
Super-resolution images detected apparent enrichment of LifeAct::GFP in DD spines versus the dendritic shaft. Because the actin cytoskeleton is a structural hallmark of vertebrate dendritic spines ( Cingolani and Goda, 2008), we also labeled DD neurons with the actin marker, LifeAct::GFP ( Riedl et al., 2008). We used Airyscan imaging, a type of super-resolution microscopy ( Korobchevskaya et al., 2017), to detect spine-like projections on the ventral processes of adult DD neurons labeled with a cytosolic mCherry marker ( Figure 1B). In the adult, Dorsal D (DD) class GABAergic motor neurons extend axons to innervate dorsal muscles and receive cholinergic input at ventral dendrites ( Figure 1A). Here, we have adopted a systematic approach to demonstrate that spine-like structures in GABAergic motor neurons (DD and VD) exhibit the salient hallmarks of dendritic spines. Finally, recent reports used light microscopy to show that a postsynaptic acetylcholine receptor is localized near the tips of spine-like protrusions on the DD class motor neurons that directly appose presynaptic termini ( Oliver et al., 2018 Philbrook et al., 2018). Light and electron microscopy detected similar dendritic protrusions extending from motor neurons in the nematode, Ascaris ( Angstadt et al., 1989 Stretton et al., 1978).

These include five classes of motor neurons (RMD, RME, SMD, DD, VD) and an interneuron (RIP) ( White et al., 1976 White et al., 1986). This approach revealed that a small subset of neurons displays short, spine-like protrusions.
#Motor neuron dendrite cell body serial#
elegans nervous system was originally defined by reconstruction of electron micrographs (EM) of serial sections. Although spine-like protrusions have been reported for invertebrate neurons ( Leiss, 2008 Petralia et al., 2016), few studies ( Bushey et al., 2011 Scott et al., 2003) have rigorously determined if these structures share functional features with vertebrate spines. For example, spine morphology and density are regulated by neural activity in plastic responses that are strongly correlated with learning and memory ( Kozorovitskiy et al., 2005 Moser et al., 1997). These dendritic ‘spines’ were originally described by Ramon y Cajal ( Yuste, 2015) and are now recognized as key functional components of neural circuits. The majority of excitatory synapses in the mammalian brain feature short, local protrusions from postsynaptic dendrites that respond to presynaptic neurotransmitter release ( Rochefort and Konnerth, 2012). elegans genetics and live-cell imaging for fundamental studies of dendritic spine morphogenesis and function.
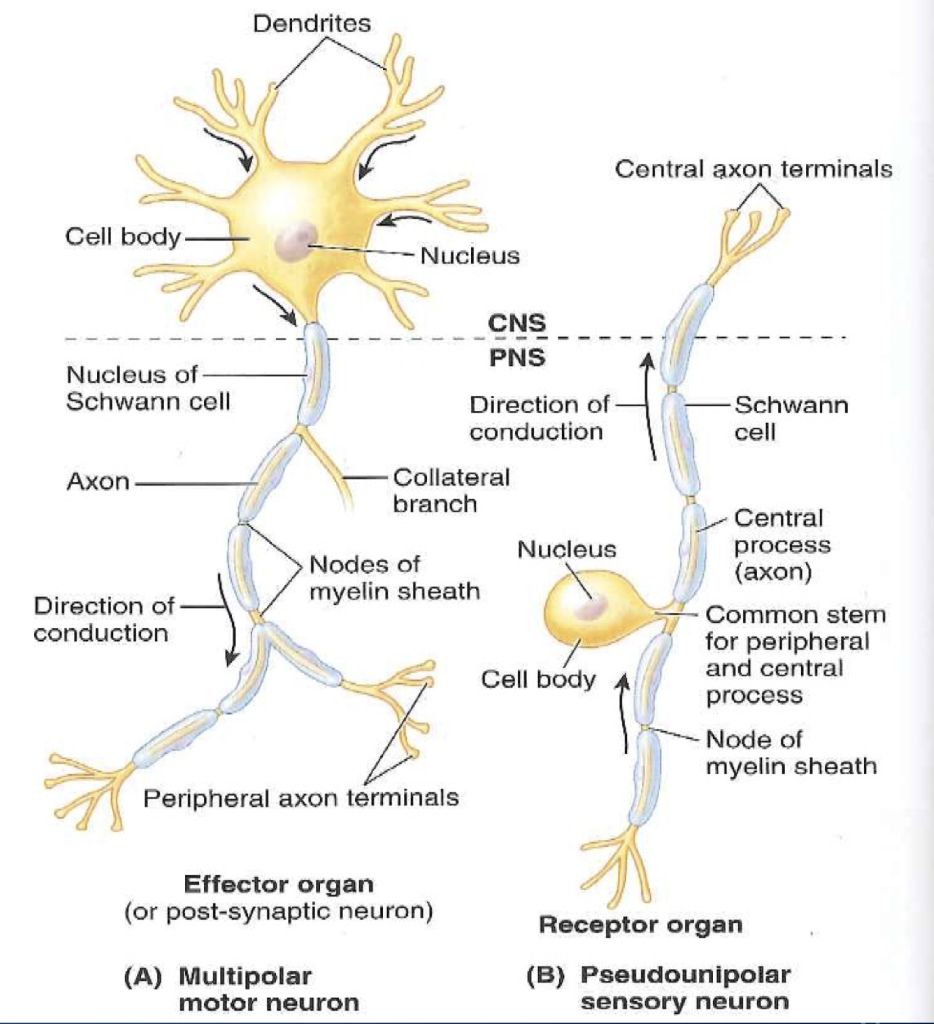
These studies provide a solid foundation for a new experimental paradigm that exploits the power of C. elegans motor neurons have functional dendritic spines that: (1) are structurally defined by a dynamic actin cytoskeleton (2) appose presynaptic dense projections (3) localize ER and ribosomes (4) display calcium transients triggered by presynaptic activity and propagated by internal Ca ++ stores (5) respond to activity-dependent signals that regulate spine density. Here, we used super-resolution microscopy, electron microscopy, live-cell imaging and genetics to show that C. elegans (Philbrook et al., 2018), suggesting that the experimental advantages of smaller model organisms could be exploited to study the biology of dendritic spines. However, spine-like protrusions have been reported in C. Most studies of spine function have focused on the mammalian nervous system. The branching in dendrites helps to increase the surface area for reception.Dendritic spines are specialized postsynaptic structures that transduce presynaptic signals, are regulated by neural activity and correlated with learning and memory.Synapses enable the dendrites from a single neuron to interact and receive signals from many other neurons.Dendrites are branched structures in neurons that extend away from the cell body to receive messages from other neurons at synapses not all neurons have dendrites.Dendrites can have small bulges called dendritic spines, which further increase surface area for possible synaptic connections.Ĭellular structure of neurons : Neurons have more specialized structures, including dendrites and axons. While some neurons have no dendrites, other types of neurons have multiple dendrites. The branching helps to increase the surface area for reception. Dendrites are tree-like structures (branched) that extend away from the cell body to receive messages from other neurons at a neuromuscular junction called synapses. Dendrites are branched structures that extend away from the cell body and carry signals toward the soma.Īside from the nucleus and other organelles, neurons contain unique structures for receiving and sending the electrical signals making neural communication possible.


 0 kommentar(er)
0 kommentar(er)
